Cell, Protein, Membrane Purification & Isolation
Protein Purification & Isolation
Easily extract, purify, clean up, and concentrate your proteins of interest with our kits, reagents, and devices. These products are optimized for a wide range of tissue and cell types and compatible with a broad range of protein purification resins and formats, which offer more choices and better protein recovery. Desalt, buffer exchange, remove contaminants and concentrate proteins with our secure and efficient devices and resins.
What do we offer?
- Protein Purification & Isolation
– Cell Lysis and Fractionation
- Detergents for Protein Solubilization
- Organelle Isolation
- Organelle Isolation Using Magnetic Beads
– Subcellular Fractionation
– Protease and Phosphatase Inhibition
- Protease Inhibitors
- Phosphatase Inhibitors
- Combined Protease and Phosphatase Inhibitors
– Cell Lysis (Total Protein Extraction)
– B-PER™ Bacterial Cell Lysis Reagents
Cell Lysis and Fractionation
Our tissue lysis, cell lysis, and cell fractionation products are optimized for sample type. The lysates or fractions collected are compatible with a wide range of downstream applications.
Features include:
- High protein yields from cells or tissues
- Gentle formulations that preserve protein activity for downstream assays
- Directly compatible with protein assays, immunoprecipitation, immunoassays, western blotting, EMSA, and enzyme assays
- Validated using multiple tissue types and cell lines
- Eliminates the need for mechanical cell disruption
|
Sample type |
Fraction of interest |
Organelle type |
Membrane disruption, protein binding and solubilization
Generally, moderate concentrations of mild (i.e., nonionic) detergents compromise the integrity of cell membranes, thereby facilitating lysis of cells and extraction of soluble protein, often in native form. Using certain buffer conditions, various detergents effectively penetrate between the membrane bilayers at concentrations sufficient to form mixed micelles with isolated phospholipids and membrane proteins.
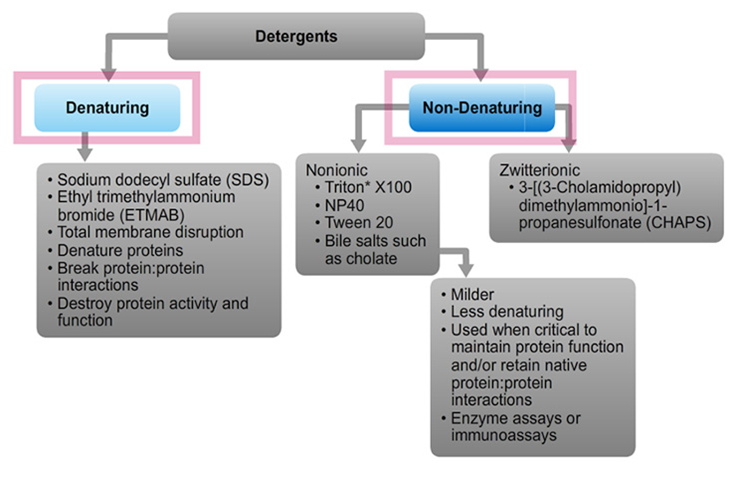
Denaturing detergents such as SDS bind to both membrane (hydrophobic) and non-membrane (water-soluble, hydrophilic) proteins at concentrations below the CMC (i.e., as monomers). The reaction is equilibrium driven until saturated. Therefore, the free concentration of monomers determines the detergent concentration. SDS binding is cooperative (i.e., the binding of one molecule of SDS increases the probability that another molecule of SDS will bind to that protein) and alters most proteins into rigid rods whose length is proportional to molecular weight.
Non-denaturing detergents such as Triton X-100 have rigid and bulky nonpolar heads that do not penetrate into water-soluble proteins; consequently, they generally do not disrupt native interactions and structures of water-soluble proteins and do not have cooperative binding properties. The main effect of non-denaturing detergents is to associate with hydrophobic parts of membrane proteins, thereby conferring miscibility to them.
At concentrations below the CMC, detergent monomers bind to water-soluble proteins. Above the CMC, binding of detergent to proteins competes with the self-association of detergent molecules into micelles. Consequently, there is effectively no increase in protein-bound detergent monomers with increasing detergent concentration beyond the CMC.
Detergent monomers solubilize membrane proteins by partitioning into the membrane bilayer. With increasing amounts of detergents, membranes undergo various stages of solubilization. The initial stage is lysis or rupture of the membrane. At detergent:membrane lipid molar ratios of 0.1:1 through 1:1, the lipid bilayer usually remains intact but selective extraction of some membrane proteins occurs. Increasing the ratio to 2:1, solubilization of the membrane occurs, resulting in mixed micelles. These include phospholipid–detergent micelles, detergent–protein micelles, and lipid–detergent–protein micelles. At a ratio of 10:1, all native membrane lipid:protein interactions are effectively exchanged for detergent:protein interactions.
Detergents for Protein Solubilization
Detergents are amphipathic molecules containing both a nonpolar "tail" having aliphatic or aromatic character, and a polar "head." The ionic character of the polar head group forms the basis for broad classification of detergents; they may be ionic (charged, either anionic or cationic), nonionic (uncharged) or zwitterionic (having both positively and negatively charged groups, but with a net charge of zero).
Detergents are frequently used in cell lysis reagent formulation, for protein solubilization procedures, in wash buffers for ELISA, and for other protein research methods. Our detergents are available in three types of packaging.
Thermo Scientific™ Pierce™ Surfact-Amps™ detergents are highly purified, precisely diluted (10%) formulations that are ideal for applications or assays that are sensitive to contaminants that are present in unpurified detergents. We test every batch to insure that our detergents contain <1.0 µeq/mL peroxides and carbonyls and package them under nitrogen, to prevent oxidization during storage.
- Superior quality—lower measurable contaminant levels than other leading vendors
- Accurate—precise 10% detergent solution in ultrapure water
- Easy-to-use—solution is simple to dispense and dilute for use
- Exceptionally pure—less than 1.0 µeq/mL peroxides and carbonyls
Properties of common detergents
|
Detergent |
Description |
Aggregation Number |
Micelle MW |
MW |
CMC (mM) |
CMC % w/v |
Cloud Point (°C) |
Dialyzable |
|
Nonionic |
140 |
90,000 |
647 |
0.24 |
0.0155 |
64 |
No |
|
|
Nonionic |
— |
— |
537 |
0.21 |
0.0113 |
23 |
No |
|
|
Nonionic |
149 |
90,000 |
617 |
0.29 |
0.0179 |
80 |
No |
|
|
Nonionic |
40 |
49,000 |
1225 |
0.09 |
0.1103 |
>100 |
No |
|
|
Nonionic |
70 |
82,000 |
1120 |
0.077 |
0.0086 |
>100 |
No |
|
|
Nonionic |
— |
— |
1228 |
0.06 |
0.0074 |
95 |
No |
|
|
Nonionic |
60 |
76,000 |
1310 |
0.012 |
0.0016 |
— |
No |
|
|
Nonionic |
27 |
8,000 |
292 |
23-25 |
0.6716-0.7300 |
>100 |
Yes |
|
|
Nonionic |
— |
— |
308 |
9 |
0.2772 |
>100 |
Yes |
|
|
Anionic |
62 |
18,000 |
288 |
6-8 |
0.1728-2304 |
>100 |
Yes |
|
|
Zwitterionic |
10 |
6,149 |
615 |
8-10 |
0.4920-0.6150 |
>100 |
Yes |
|
|
CHAPSO |
Zwitterionic |
11 |
6,940 |
631 |
8-10 |
0.5048 |
90 |
Yes |
Surfact-Amps detergents
|
Catalog # |
Name |
Size |
Price (EUR) |
|
50 mL |
205 |
||
|
28313 |
1 L |
2291 |
|
|
6 x 10 mL |
226 |
||
|
250 mL |
702 |
||
|
6 x 10 mL |
444 |
||
|
50 mL |
210 |
||
|
6 x 10 mL |
244 |
||
|
1 L |
2301 |
||
|
250 mL |
831 |
||
|
500 mL |
831 |
||
|
50 mL |
210 |
||
|
500 mL |
2400 |
||
|
6 x 10 mL |
241 |
||
|
50 mL |
218 |
||
|
6 x 10 mL |
241 |
||
|
500 mL |
1307 |
||
|
50 mL |
214 |
||
|
6 x 10 mL |
244 |
||
|
500 mL |
1307 |
Other detergents available in liquid and solid formats
|
Catalog # |
Name |
Size |
Price (EUR) |
|
1 g |
220 |
||
|
5 g |
716 |
||
|
CHAPS Detergent (3-((3-cholamidopropyl) dimethylammonio)-1-propanesulfonate) |
5 g |
241 |
|
|
100 g |
1714 |
||
|
100 g |
138 |
||
|
1 kg |
408 |
||
|
500 g |
358 |
